Media Center
A multimedia mosaic of moments at GIST
GIST Excellence
Research team led by Professor Soo Hyun Eom discovers in flavonoids a clue to the development of antiviral agents
- 김슬혜
- REG_DATE : 2014.06.11
- HIT : 1151
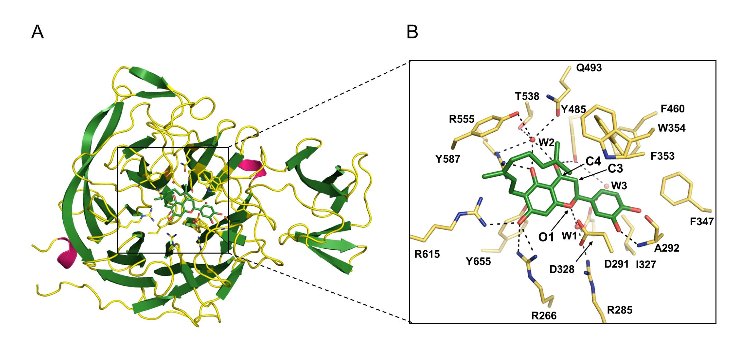
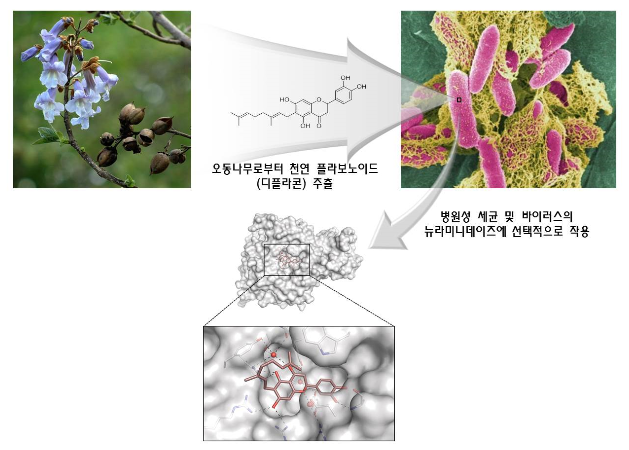
Research team led by Professor Soo Hyun Eom discovers in flavonoids a clue to the development of antiviral agents Identifies 3-dimensional structure of interaction between flavonoids treating resistance to antiviral/anti-bacteria agents and pathogenic protein □ A group of Korean researchers identified the 3-dimensional structure of the compound of natural flavonoid* extracted from paulownia coreana and neuraminidase** which is protein associated with bird flu, etc. * Flavonoid is a natural pigment which exists in plants and functions as an antioxidant o This research was led by Professor Soo Hyun Eom of GIST and Professor Park Ki Hoon of Business School (joint corresponding authors) and conducted by Mr. Lee Young Jin and Mr. Yoon Hyung Seop in the doctoral program of GIST and Ph.D Ryu Young-bae of Korea Research Institute of Bioscience and Biotechnology (joint leading authors). It was sponsored by the Senior Scientist Support Project (core research) of the Ministry of Science, ICT and Future Planning and the Steitz Center for Structural Biology of GIST. Their research finding was published by Acta Crystallographica Section D which is an international journal of crystallization structure in May (title: Structural basis of sialidase in complex with geranylated flavonoids as potent natural inhibitors) picture 1. Chemical structure of flavonoid and inhibition dose of neuraminidase (IC50) (A) basic chemical structure of a flavonoid compound extracted from paulownia coreana (B) neuraminidase activity of various flavonoids extracted from paulownia coreana : The figures marked on the right indicate the inhibition dose of neuraminidase. Diplacone, which is located at the bottom, shows the highest activation of inhibitor. □ The research team discovered that flavonoids extracted from paulownia coreana are effective in inhibiting the activation of neuraminidase and the three-dimensional structure of a compound of flavonoids and neuraminidase by X-raycrystallography. o The researchers found that flavonoids-based natural materials can also effectively inhibit neuraminidase, as well as neuraminidase inhibiters such as sialic acid-based Tamiflu and Relenza, which result is expected to provide a clue to the development of flavonoids-based antiviral agents. o They confirmed the process how diplacone* combines with the neuraminidase of clostridium which is a pathogenic germ causing peritonitis and gas gangrene at the atomic level and inhibits the function of enzyme. * Diplacone is a kind of flavonoids which consists of a flavonoid, as a main structure, and geranyl. Picture 2. Structure of a neuraminidase-flavonoid (diplacone) compound (A) 3-dimensional structure of a neuraminidase-flavonoid (diplacone) compound (green, bar-shape). β-pleated sheet (light green) of the propeller is the main structure and two short α-helixes (purple) are connected through a link (yellow). (B) Zoom-in image of neuraminidase-active area: Hydrogen bond including water molecules (red, W1,W2) contributes to interaction. □ The flavonoid compound discovered by the research team is deemed not to be effective to neuraminidase in the human body and therefore the possibility of side-effects is low. o Professor Eom said “This research finding will contribute to the development of new medicines using flavonoids-based natural ingredients to inhibit neuraminidase resistant to antiviral agents at a time when the emergency of viruses resistant to antiviral agents necessitates the development of new neuraminidase inhibitors.” Picture 3. Mimic diagram of the research finding Diplacone which is a natural flavonoid extracted from the leaves and stems of paulownia coreana (upper arrow) selectively inhibits the neuraminidase of pathogenic virus and bacteria (upper right). Based on this finding, the 3-dimentional structure of a neuraminidase-flavonoid (diplacone) compound was identified at the atomic level of definition by X-ray crystallography (bottom), thus providing a clue to the development of new medicines based on natural flavonoids to inhibit neuraminidase. 
** Neuraminidase is protein which recognizes host cells related to infection with and proliferation of various viruses and pathogenic bacteria causing bird flu, peritonitis, etc. and hence is used as a target enzyme for the development of antiviral and anti bacterial agents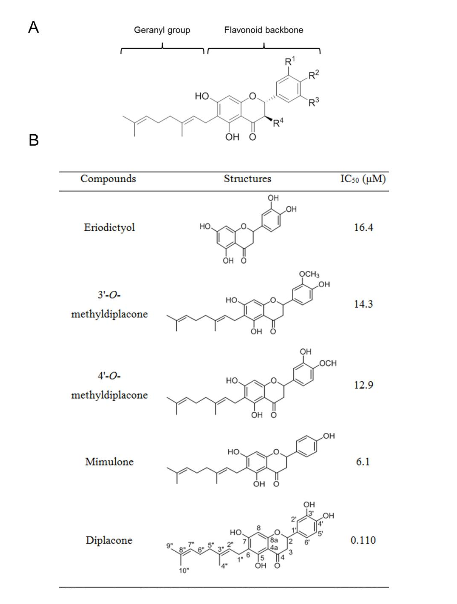
* Sialic acid is amino sugar mostly found in glycoproteins and gangliosides, and serves as a neuraminidase substrate.