Media Center
A multimedia mosaic of moments at GIST
GIST Excellence
[Press Release] GIST Professor Woo Jin Park leads an international research team to reverse cardiac fibrosis
- 엘리스 리
- REG_DATE : 2016.03.29
- HIT : 816
GIST Professor Woo Jin Park leads an
international research team to reverse cardiac fibrosis
Fibrosis is a medical condition where excessive connective tissues
form in organs, and this process occurs naturally in certain organs—such as
the kidneys, liver, and lungs—as people become older. Cardiac fibrosis is
associated with increased ventricular stiffness and diastolic dysfunction and
is an independent predictor of long-term clinical outcomes of patients with
heart failure, and heart failure is the most common cause of hospitalization in
people over the age of 65. So finding an effective treatment for cardiac fibrosis
is an important goal, especially as the world"s aging population continues to live
longer.
Professor Woo Jin Park of the School of Life Sciences at the
Gwangju Institute of Science and Technology (GIST) has led an international
research team comprised of members from the Cardiovascular Research Center of
the Icahn School of Medicine in New York, Benioff Children"s Hospital of the
University of California in San Francisco, and Paean Biotechnology of the
Chungnam National University in Daejeon, Korea.
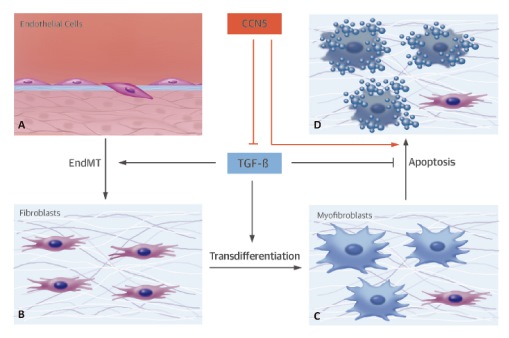
CCN5 is one of six members of the CCN (connective tissue
growth factor/cysteine-rich 61/nephroblastoma overexpressed) family, and this
family of proteins has been shown to play important roles in many processes,
including proliferation, migration, adhesion, extracellular matrix regulation,
angiogenesis, tumorigenesis, fibrosis, and implantation.
This research team examined the role of CCN5 in human heart
failure and tested whether CCN5 can reverse established CF in an experimental
model of HF induced by pressure overload. This study was performed by obtaining
human hearts from patients with end-stage heart failure. Extensive CF was
induced by applying transverse aortic constriction for 8 weeks, which was
followed by adeno-associated virus-mediated transfer of CCN5 to the heart.
Eight weeks following gene transfer, cellular and molecular effects were
examined.
The study revealed that xpression of CCN5 was significantly
decreased in failing hearts from patients with end-stage heart failure compared
to nonfailing hearts. Trichrome staining and myofibroblast content measurements
revealed that the established CF had been reversed by CCN5 gene transfer.
Anti-CF effects of CCN5 were associated with inhibition of the transforming
growth factor beta signaling pathway. CCN5 significantly inhibited
endothelial-mesenchymal transition and fibroblast-to-myofibroblast
transdifferentiation, which are 2 critical processes for CF progression, both
in vivo and in vitro. In addition, CCN5 induced apoptosis in myofibroblasts,
but not in cardiomyocytes or fibroblasts, both in vivo and in vitro. CCN5
provoked the intrinsic apoptotic pathway specifically in myofibroblasts, which
may have been due the ability of CCN5 to inhibit the activity of NFκB, an
antiapoptotic molecule.
This study concluded that CCN5 can reverse established CF by
inhibiting the generation of and enhancing apoptosis of myofibroblasts in the
myocardium. CCN5 may provide a novel platform for the development of targeted
anti-CF therapies.

The results of the study were published on April 5, 2016, in
the online edition of the Journal of the American College of Cardiology in a
paper entitled "Matricellular Protein CCN5 Reverses Established Cardiac
Fibrosis" and authored by Dongtak Jeong, Min-Ah Lee, Yan Li, Dong Kwon
Yang, Changwon Kho, Jae Gyun Oh, Gyeongdeok Hong, Ahyoung Lee, Min Ho Song,
Thomas J. LaRocca, Jiqiu Chen, Lifan Liang, Shinichi Mitsuyama, Valentina
D"Escamard, Jason C. Kovacic, Tae Hwan Kwak, Roger J. Hajjar, and Woo Jin Park.
Professor Woo Jin Park said, "Fibrosis is known to
cause diseases in various organs in addition to the heart, such as the kidney,
livers, and lungs. This study showed that it is possible to reverse cardiac
fibrosis, and we expect these results to contribute significantly to fibrosis
research and treatment options."