Media Center
A multimedia mosaic of moments at GIST
GIST Excellence
[Press Release] Existing hypothesis on the principles of endoplasmic reticulum generation has been overturned
- 엘리스 리
- REG_DATE : 2015.08.28
- HIT : 772
Existing hypothesis on the principles of
endoplasmic reticulum generation has been overturned
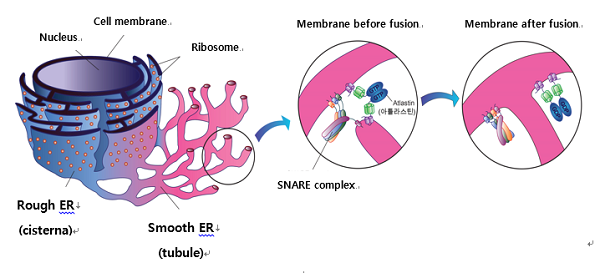
Figure 1. ER membrane fusion and mesh structure by SNARE complex. ER forms a net like interconnected structure by membrane fusion between tubules. At this time, atlastin protein mediates the physical contact between tubules and help SNARE to move to a membrane fusion area. Finally, the process completes the fusion and ER forms a net like structure.
□ Minister Yanghee Choi of the Ministry of Science, ICT and Future Planning said, “Korean researchers have opened up the possibility of developing medicines for rare incurable diseases, such as the hereditary spastic paraplegia, by recently identifying the principles of eukaryotic endoplasmic reticulum (ER) generation.”
□ GIST Professor Youngsoo Jun and Ph.D candidates Miriam Lee and Young Joon Ko researched identifying membrane fusion of endoplasmic reticulum (ER) with the support from the ‘Integrative Aging Research Center’s Silver Health Biotechnology Development Project’. The paper has been published in The Journal of Cell Biology on August 3, 2015.
○- Title: SNAREs support atlastin-mediated homotypic ER fusion in Saccharomyces cerevisiae
- Authors: Young Soo Jun (Corresponding author, GIST School of Life Sciences), Miriam Lee, Young Joon Ko (Co-authors, GIST Ph.D. candidates and researchers)
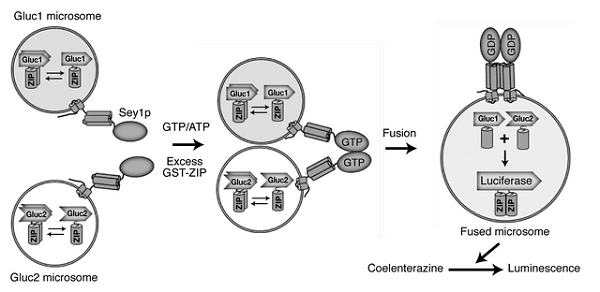
Figure 2. Isolated and purified yeast (Saccharomyces cerevisiae) ER fusion analysis system. Membrane fusion between ER activates yeast (induce luminescence) therefore, measuring yeast activities, one can measure the membrane fusion.
Normal ER tubule structure Mutated SNARE ER
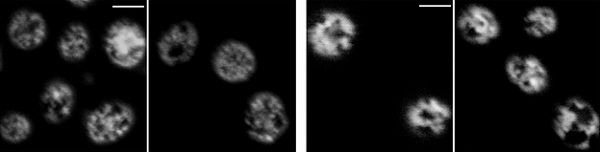
Figure 3. Defected ER tubule structure due to the SNARE mutation. In normal yeast, mesh structure of ER tubule is clearly observed but when atlastin and SNARE protein are removed, the mesh structure is lost.
Dynamin-like GTPases of the atlastin family are thought to mediate homotypic endoplasmic reticulum (ER) membrane fusion; however, the underlying mechanism remains largely unclear. Here, we developed a simple and quantitative in vitro assay using isolated yeast microsomes for measuring yeast atlastin Sey1p-dependent ER fusion. Using this assay, we found that the ER SNAREs Sec22p and Sec20p were required for Sey1p-mediated ER fusion. Consistently, ER fusion was significantly reduced by inhibition of Sec18p and Sec17p, which regulate SNARE-mediated membrane fusion. The involvement of SNAREs in Sey1p-dependent ER fusion was further supported by the physical interaction of Sey1p with Sec22p and Ufe1p, another ER SNARE. Furthermore, our estimation of the concentration of Sey1p on isolated microsomes, together with the lack of fusion between Sey1p proteoliposomes even with a 25-fold excess of the physiological concentration of Sey1p, suggests that Sey1p requires additional factors to support ER fusion in vivo. Collectively, our data strongly suggest that SNARE-mediated membrane fusion is involved in atlastin-initiated homotypic ER fusion.

Professor Youngsoo Jun and Ph.D. candidates Miriam Lee and Young Joon Ko of the School of Life Sciences
Professor Youngsoo Jun said, “This research has tremendous significance to refute an existing hypothesis and proposes a new hypothesis for the principle of ER generation. It is expected that this newly developed ER membrane fusion analysis system would be useful to help identify future ER membrane fusion processes and ER structure formation principles. Moreover, the research will be able to provide theoretical clues in the development of incurable disease treatments, such as a hereditary spastic paraplegia, which is caused by structural defects of ER.”