Media Center
A multimedia mosaic of moments at GIST
GIST Excellence
[Press release] Professor Sung-Gyoo Park"s research team uncovers cereblon"s role in regulating T-cell activation
- 엘리스 리
- REG_DATE : 2016.08.08
- HIT : 1018
Professor Sung-Gyoo Park"s research team uncovers
cereblon"s role in regulating T-cell activation

From left: Professor Sung-Gyoo Park, Ph.D. student Jung-Ah Kang, and Ph.D. student Sand-Heon Park
Cereblon (CRBN) is a thalidomide-binding protein that can be found in the cytoplasm of cells as well as various other cellular membranes and structures, yet its role in T-cell regulation has not been well understood until a research team at the Gwangju Institute of Science and Technology (GIST) led by Professor Sung-Gyoo Park of the School of Life Science recently deleted the Crbn gene from mice to study the physiological role of CRBN, which the research team has identified as an important regulator of T-cell activation. The research team concluded that CRBN deficiency does not affect T-cell development, but it does increase T-cell activation.
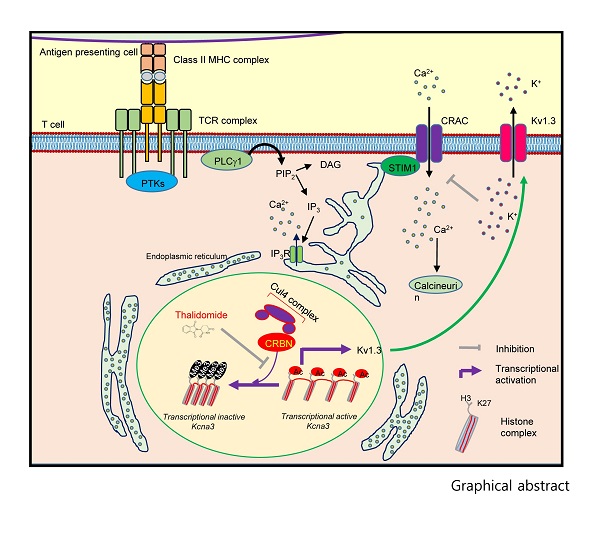
Figure 1. The role of CRBN in the CD4+ T-cell activation process is shown.
T-cells play a crucial role in a person"s adaptive immune system, and CRBN normally prevents hyperactivation of T-cells by repressing the expression of the Kv1.3 potassium channel by binding to the chromosomal regions adjacent to the Kcna3 locus thereby limiting calcium influx into the T-cells by histone modifications of Kcna3 chromatin. However, if CRBN is not present, then there is an upregulation of Kv1.3 expression that causes an increase in potassium flux that, in turn, results in an increase in calcium flux during T-cell activation.
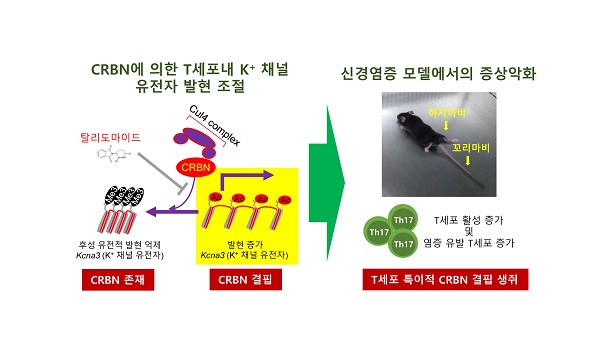
Figure 2. The mouse model for experimental autoimmune encephalomyelitis for Crbn-deficient mice is shown.
When the researchers tested the mouse model for experimental autoimmune encephalomyelitis (EAE) with Crbn-deficient mice, the Crbn-deficient mice exhibited worsening and sustained symptoms of EAE. Because EAE is an animal model that is used to study brain inflammation and diseases, such as multiple sclerosis, the results of this research may offer important insights on regulating CRBN levels to provide new therapeutic treatment options for various autoimmune conditions and diseases.
Their paper entitled "Epigenetic regulation of Kcna3-encoding Kv1.3 potassium channel by cereblon contributes to regulation of CD4+ T-cell activation" was authored by Jung-Ah Kang, Sang-Heon Park, Sang Phil Jeong, Min-Hee Han, Cho-Rong Lee, Kwang Min Lee, Namhee Kim, Mi-Ryoung Song, Murim Choi, Michael Ye, Guhung Jung, Won-Woo Lee, Soo Hyun Eom, Chul-Seung Park, and Sung-Gyoo Park and published on August 2, 2016, in PNAS.
Professor Sung-Gyoo Park said, "By manipulating the relevant Crbn genes, our research has discovered the important role of CRBN in regulating T-cell activation, and, by doing this, we have also demonstrated a new and novel way to treat T-cell related immune diseases by controlling the level of CRBN with gene therapy."